Abstract
Background: Increasing evidence has pointed out that inherited NUDT15 variants are strongly associated with 6-mercaptopurine (6-MP) toxicities that may require therapy interruption in diseases such as acute lymphoblastic leukemia (ALL), inflammatory bowel diseases, and rheumatic diseases. However, the consequences and costs incurred from treating 6-MP-induced toxicities due to inherited NUDT15 variants are not well explored so far.
Objective: This study aims to retrospectively describe the infection rate and medical costs incurred from the treatment of 6-MP toxicities in the absence of NUDT15 genotype-guided dose adjustment in childhood ALL patients who received systemic therapy in our institution, and to compare infection rate and costs between individuals with and without NUDT15 variants.
Methods: The study population involved childhood ALL patients below 14 years of age, who were enrolled in CCCG-ALL-2015 (ChiCTR-IPR-14005706) treatment. Patients were categorized into low-, intermediate- and high-risk based on clinical presentation, cytogenetic abnormalities and therapeutic minimal residual disease level. 6-MP was administered at an initial dose of 50mg/m2, and was modified at subsequent administrations based on presenting toxicities and physicians' clinical discretion. Data on infections, as well as medical costs attributable to 6-MP induced toxicities (infections, rash, mucositis, diarrhea etc.), were retrieved retrospectively and analyzed in chronological phases of treatment based on the protocol. Costs were converted to US dollars. Descriptive statistics was used to summarize all clinical and cost data. Independent t-test was used to compare clinical cost data between patients with and without NUDT15 variants for overall, as well as for each stage of therapy.
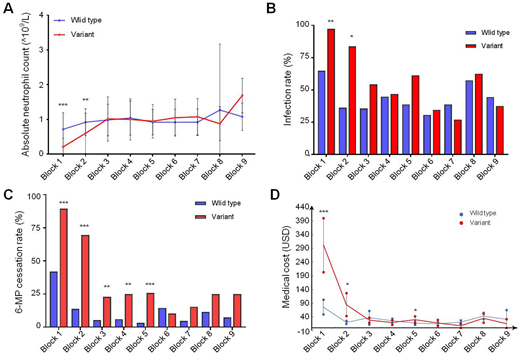
Results: A total of 144 patients were included in this study, of whom 45 patients (31%) were found to have NUDT15 variants (13 cases with exon 1 variant, 16 cases with concurrence of exon 1 and 3 variants, and 16 cases with exon 3 variants) after retrospective screening. However, we did not identify patients with TPMT susceptible variants in our cohort. Patients with NUDT15 inherited variants were more susceptible to myeloisuppression (Figure 1A, variants with mean ANC [95% CI] 0.21 [0.12-0.29]) vs wild type 0.71 [0.61-.81], P<0.0001) and infection (Figure 1B, P<0.0001), especially in those with rs116855232 T/T homozygous genotype. Due to severe myelosuppression and infections, systemic 6-MP administration was frequently interrupted within the patients with NUDT15 variants (Figure 1C, P<0.0001). Consequently, the costs incurred from 6-MP toxicities was almost twice higher in the group with NUDT15 variants, as compared to the wild type group (mean cost per patient [95% CI] 417.9 [303.0-532.8] vs 199.1 [160.6-237.7], P=0.001). To highlight, medical costs incurred from toxicity management were significantly higher in the group with NUDT15 variants during the first block (P<0.0001) and second block (P=0.005) of treatment (Figure 1D). In subsequent stages of treatment, there is no difference in costs incurred between both groups (Figure 1D).
Conclusion: We found that patients with NUDT15 variants demonstrated increased susceptibility to severe infections. They also incurred higher medical costs associated with the management of 6-MP toxicities without prior NUDT15-guided dose adjustment, particularly during the early maintenance phase. These preliminary findings suggest that routine pre-treatment screening for NUDT15 variants and early NUDT15-guided 6-MP dose modification may potentially reduce the incidence and cost of managing 6-MP toxicities. A larger prospective cohort should be conducted to validate our findings and thus benefit the children with ALL.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.
This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal